We remember from our school chemistry course that every element has its own specific atomic number. It is the same as the number of protons that the atom of each element has, so sometimes atomic number is called proton number. It is always the whole number and it ranges from 1 to 118, according to the number of the element in the Periodic Table. This number can be really important and something essential to know, in relation to a certain chemical element which is the issue of our interest at the moment.
Why is this so? Why is the atomic number so important? First of all, it is the number that makes elements different from one another as it shows the number of protons in their nuclei. Also, knowing the atomic number of an element can give us an idea about the position of the element in the Periodic Table. Atomic number of an element never changes: for example, the atomic number of oxygen is always 8, and the atomic number of Chlorine is always 18. The atomic number is marked with the symbol Z, taken from a German word zahl (or atomzahl, which is 'atomic number' in German).
An element's atomic number is the number of protons in the nucleus of a single atom of that element. The atomic number of an element or isotope cannot change, so you can use the atomic number to help figure out other characteristics, such as the number of electrons and neutrons in an atom. Part 1 Finding the Atomic Number. The atomic mass of an element is the average mass of the atoms of an element measured in atomic mass unit (amu, also known as daltons, D).The atomic mass is a weighted average of all of the isotopes of that element, in which the mass of each isotope is multiplied by the abundance of that particular isotope.
This website is created for those who need to know the atomic number of a central chemical element. By using our website, you can do it in just one click and receive short and correct information on this matter. There is also some extra summary on every each chemical element which can be found at our website, including the atomic weight of each element, as well as physical and chemical properties of every element and its importance. Use this website at any time when you need to get fast and precise information about atomic or proton number of chemical elements.
List of chemical elements in periodic table with atomic number, chemical symbol and atomic weight. You can sort the elements by clicking on the table headers. Please click on the element name for complete list of element properties.
| Atomic Number | Chemical Symbol | Element Name | Atomic Weight (u) |
|---|---|---|---|
| 1 | H | Hydrogen | 1.008 |
| 2 | He | Helium | 4.003 |
| 3 | Li | Lithium | 6.94 |
| 4 | Be | Beryllium | 9.012 |
| 5 | B | Boron | 10.81 |
| 6 | C | Carbon | 12.011 |
| 7 | N | Nitrogen | 14.007 |
| 8 | O | Oxygen | 15.999 |
| 9 | F | Fluorine | 18.998 |
| 10 | Ne | Neon | 20.18 |
| 11 | Na | Sodium | 22.99 |
| 12 | Mg | Magnesium | 24.305 |
| 13 | Al | Aluminium | 26.982 |
| 14 | Si | Silicon | 28.085 |
| 15 | P | Phosphorus | 30.974 |
| 16 | S | Sulfur | 32.06 |
| 17 | Cl | Chlorine | 35.45 |
| 18 | Ar | Argon | 39.948 |
| 19 | K | Potassium | 39.098 |
| 20 | Ca | Calcium | 40.078 |
| 21 | Sc | Scandium | 44.956 |
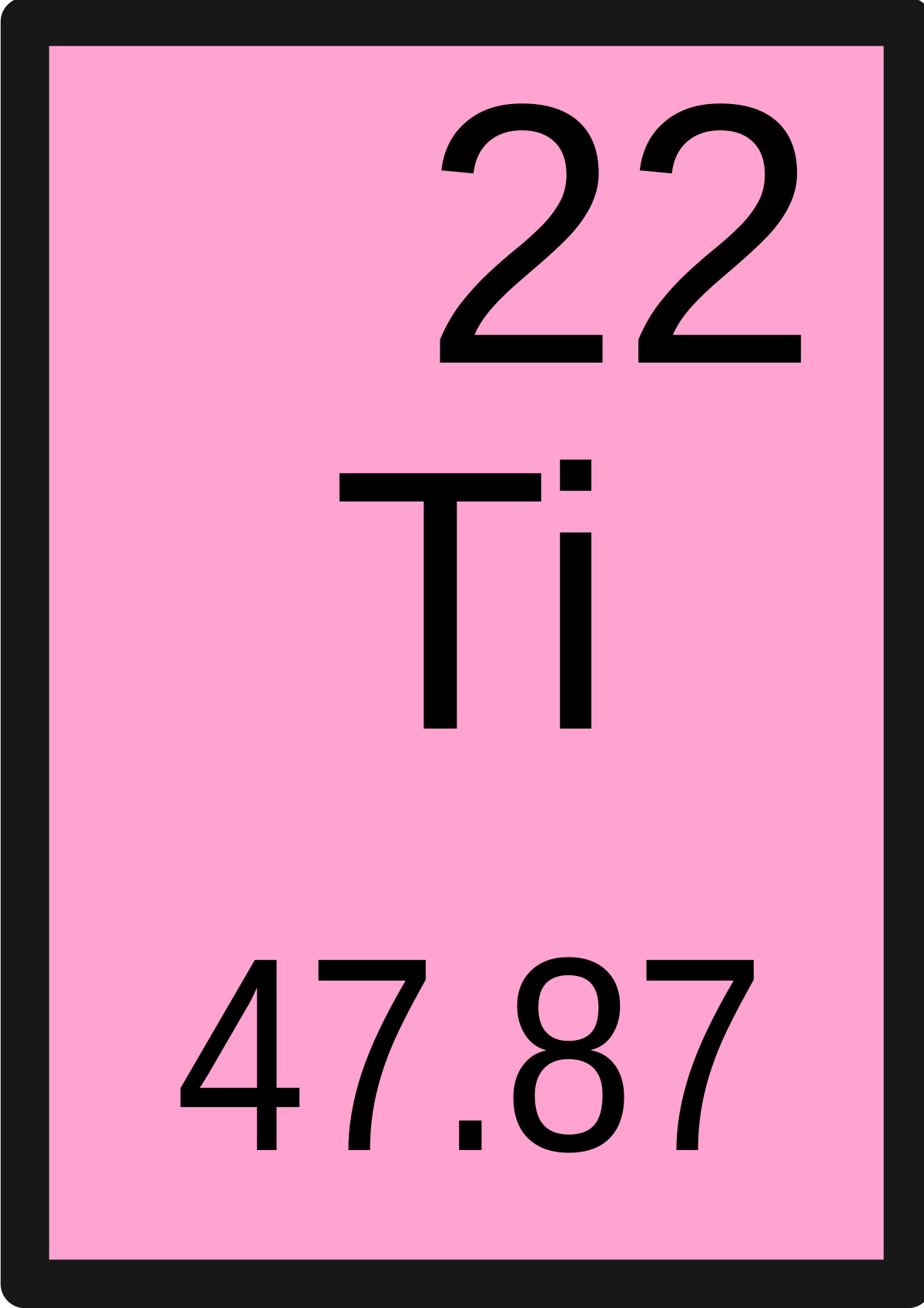
| 22 | Ti | Titanium | 47.867 |
| 23 | V | Vanadium | 50.942 |
| 24 | Cr | Chromium | 51.996 |
| 25 | Mn | Manganese | 54.938 |
| 26 | Fe | Iron | 55.845 |
| 27 | Co | Cobalt | 58.933 |
| 28 | Ni | Nickel | 58.693 |
| 29 | Cu | Copper | 63.546 |
| 30 | Zn | Zinc | 65.38 |
| 31 | Ga | Gallium | 69.723 |
| 32 | Ge | Germanium | 72.63 |
| 33 | As | Arsenic | 74.922 |
| 34 | Se | Selenium | 78.971 |
| 35 | Br | Bromine | 79.904 |
| 36 | Kr | Krypton | 83.798 |
| 37 | Rb | Rubidium | 85.468 |
| 38 | Sr | Strontium | 87.62 |
| 39 | Y | Yttrium | 88.906 |
| 40 | Zr | Zirconium | 91.224 |
| 41 | Nb | Niobium | 92.906 |
| 42 | Mo | Molybdenum | 95.95 |
| 43 | Tc | Technetium | 98 |
| 44 | Ru | Ruthenium | 101.07 |
| 45 | Rh | Rhodium | 102.906 |
| 46 | Pd | Palladium | 106.42 |
| 47 | Ag | Silver | 107.868 |
| 48 | Cd | Cadmium | 112.414 |
| 49 | In | Indium | 114.818 |
| 50 | Sn | Tin | 118.71 |
| 51 | Sb | Antimony | 121.76 |
| 52 | Te | Tellurium | 127.6 |
| 53 | I | Iodine | 126.904 |
| 54 | Xe | Xenon | 131.293 |
| 55 | Cs | Caesium | 132.905 |
| 56 | Ba | Barium | 137.327 |
| 57 | La | Lanthanum | 138.905 |
| 58 | Ce | Cerium | 140.116 |
| 59 | Pr | Praseodymium | 140.908 |
| 60 | Nd | Neodymium | 144.242 |
| 61 | Pm | Promethium | 145 |
| 62 | Sm | Samarium | 150.36 |
| 63 | Eu | Europium | 151.964 |
| 64 | Gd | Gadolinium | 157.25 |
| 65 | Tb | Terbium | 158.925 |
| 66 | Dy | Dysprosium | 162.5 |
| 67 | Ho | Holmium | 164.93 |
| 68 | Er | Erbium | 167.259 |
| 69 | Tm | Thulium | 168.934 |
| 70 | Yb | Ytterbium | 173.045 |
| 71 | Lu | Lutetium | 174.967 |
| 72 | Hf | Hafnium | 178.49 |
| 73 | Ta | Tantalum | 180.948 |
| 74 | W | Tungsten | 183.84 |
| 75 | Re | Rhenium | 186.207 |
| 76 | Os | Osmium | 190.23 |
| 77 | Ir | Iridium | 192.217 |
| 78 | Pt | Platinum | 195.084 |
| 79 | Au | Gold | 196.967 |
| 80 | Hg | Mercury | 200.592 |
| 81 | Tl | Thallium | 204.38 |
| 82 | Pb | Lead | 207.2 |
| 83 | Bi | Bismuth | 208.98 |
| 84 | Po | Polonium | 209 |
| 85 | At | Astatine | 210 |
| 86 | Rn | Radon | 222 |
| 87 | Fr | Francium | 223 |
| 88 | Ra | Radium | 226 |
| 89 | Ac | Actinium | 227 |
| 90 | Th | Thorium | 232.038 |
| 91 | Pa | Protactinium | 231.036 |
| 92 | U | Uranium | 238.029 |
| 93 | Np | Neptunium | 237 |
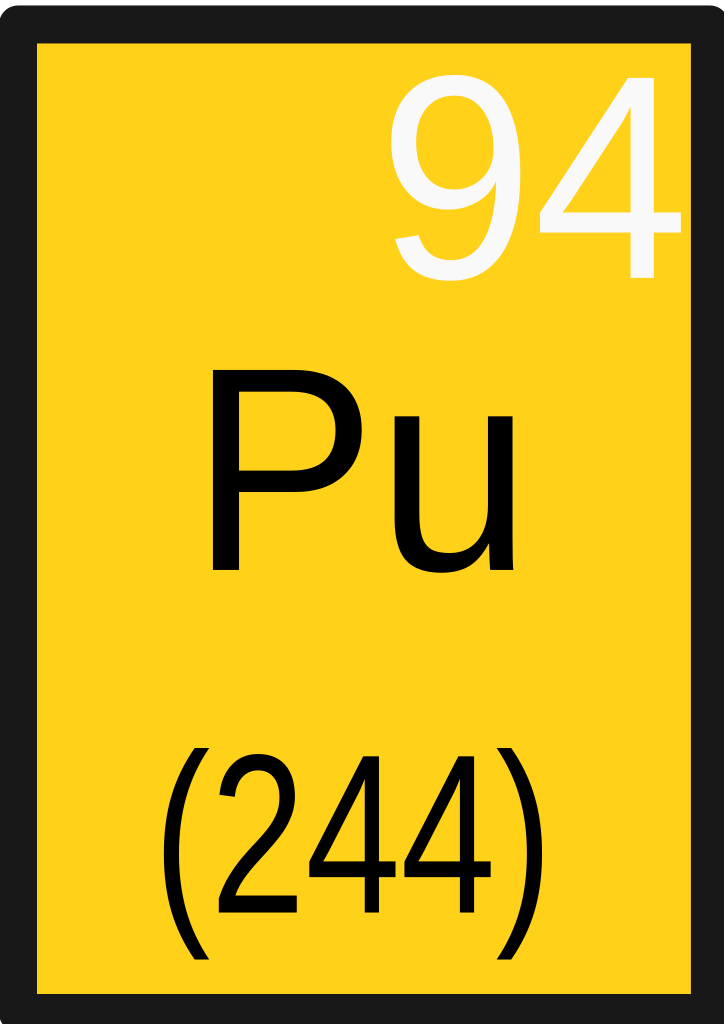
| 94 | Pu | Plutonium | 244 |
| 95 | Am | Americium | 243 |
| 96 | Cm | Curium | 247 |
| 97 | Bk | Berkelium | 247 |
| 98 | Cf | Californium | 251 |
| 99 | Es | Einsteinium | 252 |
| 100 | Fm | Fermium | 257 |
| 101 | Md | Mendelevium | 258 |
| 102 | No | Nobelium | 259 |
| 103 | Lr | Lawrencium | 266 |
| 104 | Rf | Rutherfordium | 267 |
| 105 | Db | Dubnium | 268 |
| 106 | Sg | Seaborgium | 269 |
| 107 | Bh | Bohrium | 270 |
| 108 | Hs | Hassium | 277 |
| 109 | Mt | Meitnerium | 278 |
| 110 | Ds | Darmstadtium | 281 |
| 111 | Rg | Roentgenium | 282 |
| 112 | Cn | Copernicium | 285 |
| 113 | Nh | Nihonium | 286 |
| 114 | Fl | Flerovium | 289 |
| 115 | Mc | Moscovium | 290 |
| 116 | Lv | Livermorium | 293 |
| 117 | Ts | Tennessine | 294 |
| 118 | Og | Oganesson | 294 |
Lists of Elements in Periodic Table
You can also list the elements in various ordered properties with printable tables below.
Lists of Elements by Group Number in Periodic Table
» Group 1» Group 2» Group 3» Group 4» Group 5» Group 6» Group 7» Group 8» Group 9» Group 10» Group 11» Group 12» Group 13» Group 14» Group 15» Group 16» Group 17» Group 18Learning Objective
- Determine the relationship between the mass number of an atom, its atomic number, its atomic mass, and its number of subatomic particles
Key Points
- Neutral atoms of each element contain an equal number of protons and electrons.
- The number of protons determines an element’s atomic number and is used to distinguish one element from another.
- The number of neutrons is variable, resulting in isotopes, which are different forms of the same atom that vary only in the number of neutrons they possess.
- Together, the number of protons and the number of neutrons determine an element’s mass number.
- Since an element’s isotopes have slightly different mass numbers, the atomic mass is calculated by obtaining the mean of the mass numbers for its isotopes.
Terms
Atomic Number Is The Number Of Protons

- atomic massThe average mass of an atom, taking into account all its naturally occurring isotopes.
- mass numberThe sum of the number of protons and the number of neutrons in an atom.
- atomic numberThe number of protons in an atom.
Atomic Number
Neutral atoms of an element contain an equal number of protons and electrons. The number of protons determines an element’s atomic number (Z) and distinguishes one element from another. For example, carbon’s atomic number (Z) is 6 because it has 6 protons. The number of neutrons can vary to produce isotopes, which are atoms of the same element that have different numbers of neutrons. The number of electrons can also be different in atoms of the same element, thus producing ions (charged atoms). For instance, iron, Fe, can exist in its neutral state, or in the +2 and +3 ionic states.
Mass Number
An element’s mass number (A) is the sum of the number of protons and the number of neutrons. The small contribution of mass from electrons is disregarded in calculating the mass number. This approximation of mass can be used to easily calculate how many neutrons an element has by simply subtracting the number of protons from the mass number. Protons and neutrons both weigh about one atomic mass unit or amu. Isotopes of the same element will have the same atomic number but different mass numbers.
Scientists determine the atomic mass by calculating the mean of the mass numbers for its naturally-occurring isotopes. Often, the resulting number contains a decimal. For example, the atomic mass of chlorine (Cl) is 35.45 amu because chlorine is composed of several isotopes, some (the majority) with an atomic mass of 35 amu (17 protons and 18 neutrons) and some with an atomic mass of 37 amu (17 protons and 20 neutrons).
Atomic Number Is 50 Crossword
Given an atomic number (Z) and mass number (A), you can find the number of protons, neutrons, and electrons in a neutral atom. For example, a lithium atom (Z=3, A=7 amu) contains three protons (found from Z), three electrons (as the number of protons is equal to the number of electrons in an atom), and four neutrons (7 – 3 = 4).
Show SourcesAtomic Number Is 14
Boundless vets and curates high-quality, openly licensed content from around the Internet. This particular resource used the following sources:
Atomic Number Is Based On
http://www.boundless.com/
Boundless Learning
CC BY-SA 3.0.

http://en.wiktionary.org/wiki/atomic_number
Wiktionary
CC BY-SA 3.0.
http://www.boundless.com//biology/definition/atomic-mass–2
Boundless Learning
CC BY-SA 3.0.
http://en.wikibooks.org/wiki/A-level_Chemistry/OCR/Atoms,_Bonds_and_Groups/Atoms_and_Reactions/Atoms
Wikibooks
CC BY-SA 3.0.
http://cnx.org/content/m44390/latest/?collection=col11448/latest
OpenStax CNX
CC BY 3.0.




